CLINICAL DOCUMENT MANAGEMENT
Meet Marvin eTMF
Marvin eTMF supports better project management through real-time access to study documentation. Our system offers clear workflows, reporting, search and retrieval, audit-readiness at each point in time, and reduce the administrative overhead associated with running clinical trials.
NO CUMBERSOME MANUAL PREPARATION
Focus on the Clinical Trial Process
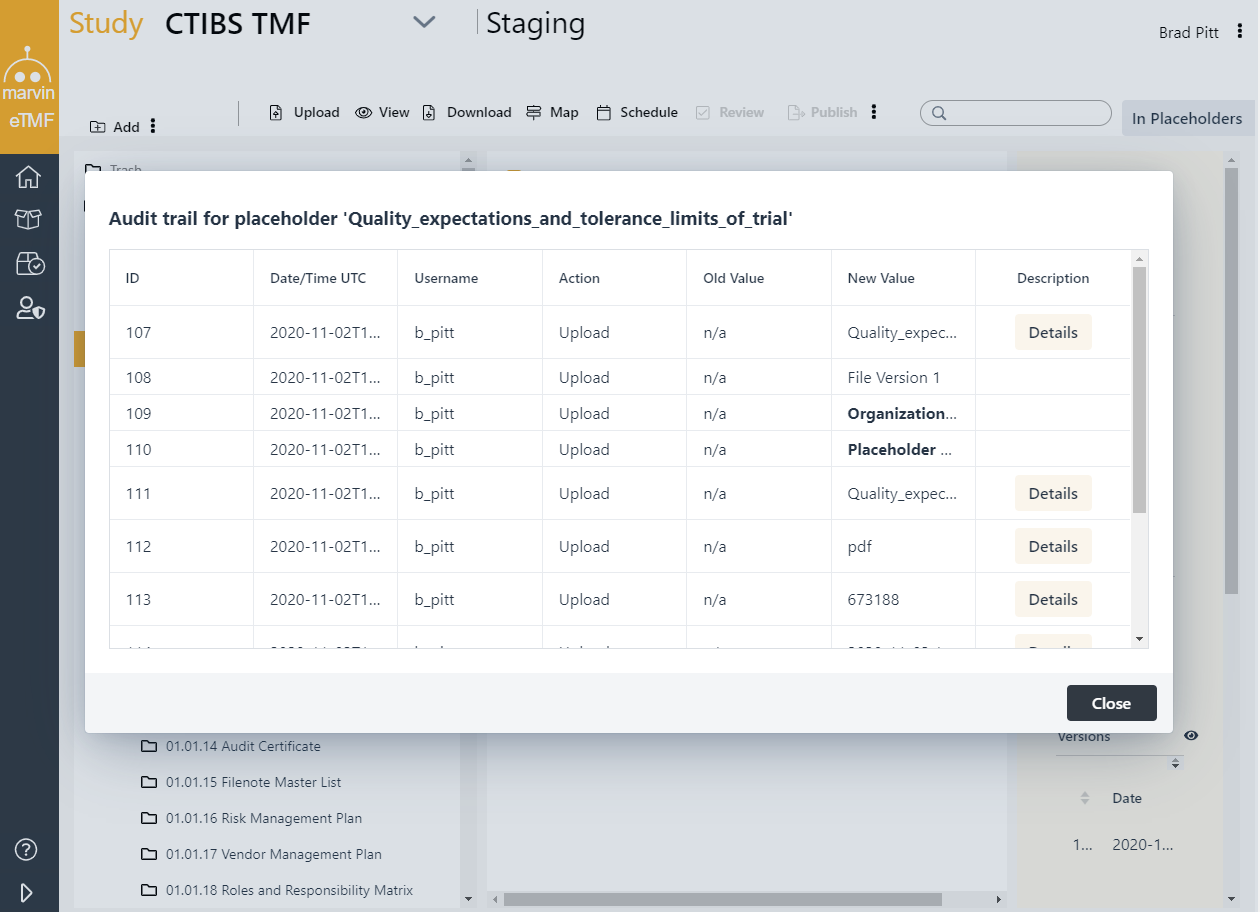
Enjoy full audit trail and versioning, Metadata recording, Search and retrieval for audit readiness e-approval submission workflow. The software incorporates fine-grained role-based access and ensures your TMF to stay inspection ready.

“The team and I are looking forward to working with you on the integration of the Marvin eTMF into your eClinical solutions as well as its future improvements.”
Electronic Master File | Marvin eTMF Capabilities
Simple and Intuitive Interface
Get up to speed in no time. Marvin eTMF makes daily work easier and more efficient. The simple and intuitive web-based interface reduces training time, lower frustration and increase efficiency. With minimal training users can exchange, and update TMF documents. CROs, Site personnel, monitors, auditors and sponsors can be trained within a few hours.
Flat Rate, No Per User Fees, Unlimited Sites
Marvin eTMF is not only attractive when it comes to functionality and usage, but also in operation. Invite anyone, unlimited users, unlimited sites. Deploys in days, not months.

Full Support of Your Own TMF Structure or The TMF Reference Model from DIA
Trial master files are not one size fits all. Marvin eTMF comes with the preconfigured, but customizable DIA Reference TMF structure, or it gives you the flexibility to use your own TMF structure for identifying and classifying trial documents. Improve inspection readiness and reduce time to set up a new site by adherence to your TMF reference model to bring order, structure, and lineage to your eTMF – helping you segment and organize any large data volume in the most efficient way.

Plotting Your Course for Filling Leading to Inspection Readiness
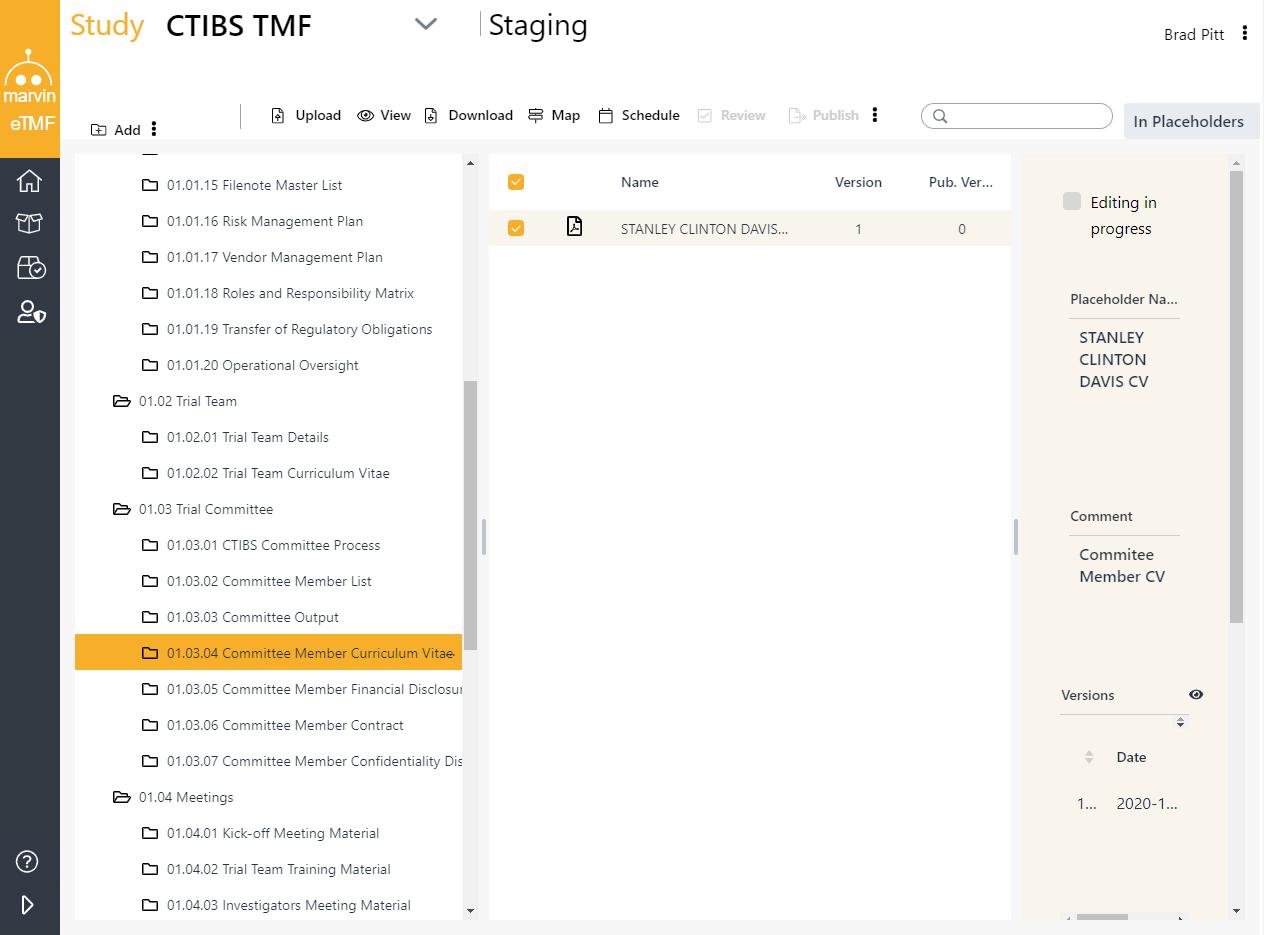
Marvin eTMF leverages digital placeholders (PHs) to automatically generate the folder structure according to a predefined file plan. A file plan is a Excel spreadsheet specifying the expected artifacts in each filing area and allows for up-to-the-moment accuracy on the compliance documentation status. Notifications for overdue files keep everybody in sync. At export, files will be named according to convention.
Fine-grained Role Based Controlled Access
Accommodate Site, Sponsor, CRO, External Users, Inspectors and auditors within one environment. Designate certain users as QC reviewers, to finalize documents as TMF-ready. The content is maintained in a secure, compliant environment with controlled access, configurable user roles, and a complete audit trail providing direct eTMF access for all study stakeholders

Ensure Regulatory Inspection Readiness
Marvin eTMF contains all components, controls and policies that are required according to 21 CFR Part 11, ICH-GCP E6 and GDPR (General Data Protection Regulation). The system is specified and validated according to the regulatory and data protection requirements.

Intuitive Search Capabilities
Marvin eTMF leverages the metadata related to your document to help locate the relevant document in a search. Metadata is the information attached to an item and captures details like the document author, file type, size, time of creation, upload time, your own comment and so on. Marvin eTMF’s search algorithms—based on filename, location, or metadata—makes searching TMF artifacts simple, accurate, and robust.

Simple Uploading and Filing
Marvin eTMF allows you to easily file the documents in the correct location in the eTMF reference model and add metadata by drag and dropping documents one by one, or in bulk.

Simple and Intuitive Interface
Get up to speed in no time. Marvin eTMF makes daily work easier and more efficient. The simple and intuitive web-based interface reduces training time, lower frustration and increase efficiency. With minimal training users can exchange, and update TMF documents. CROs, Site personnel, monitors, auditors and sponsors can be trained within a few hours.

Flat Rate, No Per User Fees, Unlimited Sites
Marvin eTMF is not only attractive when it comes to functionality and usage, but also in operation. Invite anyone, unlimited users, unlimited sites. Deploys in days, not months.

Expert Ressources
Marvin eTMF is a professional and budget friendly eClinical solution for managing all your trial relevant documentation and processes. Find the licensing module for your trial.