CLINICAL DATA MANAGEMENT
Meet Marvin EDC
all therapeutic areas :: all phases :: medical devices
Today more than 1,200 studies worldwide have been managed using Marvin EDC from XClinical. Marvin EDC is one of the most comprehensive eClinical solutions including study build software, EDC, IWRS and drug management, WebPRO, coding, SDTM tabulation, risk-based monitoring, reporting, and clinical trial management.
FLEXIBLE FOR ANY TYPE OF STUDY
Easy Study Set-Up and Management
Enjoy a well built and flexible modern application to safely collect your clinical data. A versatile software that enables easy study set-up and management. Both customers and end users value the simplicity combined with complex, customizable workflows. The integrated solution comprised of various eClinical modules optimally support clinical trial processes and enable seamless data flow.
BUILT FOR GLOBAL BUSINESS
Impressive universal capabilities
Most Clinical Trials are conducted internationally spanning multiple countries or even continents. XClinical designed Marvin with a comprehensive vision of the challenges global studies bring. Beginning with a centralized database that is accessible from any web browser, the addition of not just multi-language, but every language became a standard offering. Sensitive to the fact that not all regions have internet access, Marvin also allows for double-data entry (DDE) and hybrid studies.
ALL IN ONE ECLINICAL SOFTWARE PLATFORM
Scope of Features

IWRS Randomization
The XClinical Randomization System is an IWRS (Interactive Web Response System) integrated into Marvin and does not require an interface to any other external systems. It offers open or double-blind, multi-strata, multi-group randomization based on a block list or a widely configurable range of algorithms like variance minimization. Recently we added CARA randomization to support multi-armed clinical trials.
Key Features
-
-
-
-
-
-
-
-
-
- Seamless integration with Marvin EDC
- Advanced materials management
- Offers multiple randomization methods
-
-
-
-
-
-
-
-
Drug Supply Management
Included with Marvin IWRS, the Randomization System is a materials accountability module with (investigational) medical product stock management inventory and logistics. Shipments of materials from a central stock to different sites is administered via a web based workflow including optional package verification numbers.
Key Features
-
-
-
-
-
-
-
-
-
- Automatic shipment creation based on stock levels
- Batch shipping of IMP
- Material expiry date control
-
-
-
-
-
-
-
-
WebPRO
Integrated into Marvin EDC we have a Patient Reported Outcome (PRO) module due to the increasing importance of PRO for all types of studies. Data will be captured into the same database as all eCRF forms. This facilitates real-time reporting including various wearable devices.
Key features
-
-
-
-
-
-
-
-
-
- Integrated as part of your clinical study
- Easy to design questionnaires
- Utilize existing standards from within the composer
-
-
-
-
-
-
-
-
Risk Based Monitoring (RBM)
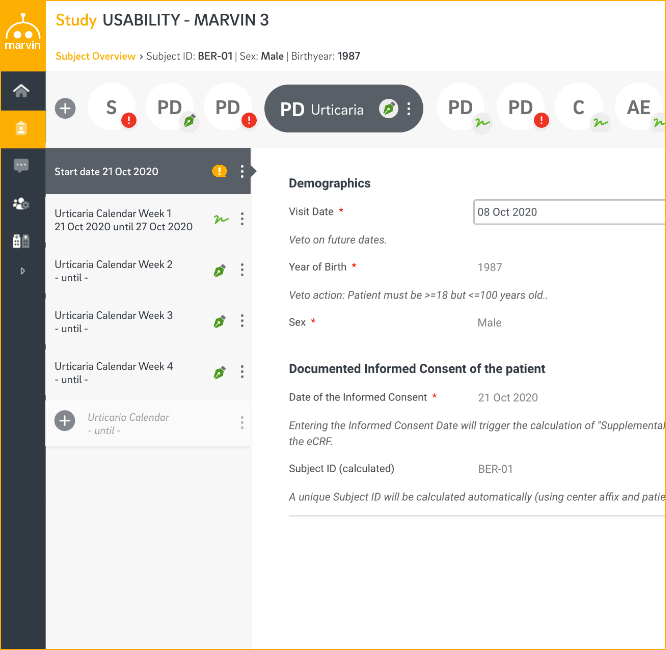
The Marvin EDC system supports the source data verification (SDV) process by distinguishing different risk levels for each specific study center. Customized verification rules are defined in a detailed Monitoring Plan containing different risk levels. It makes the system adaptable to the site and data handling workflow. The System provides an effective and cost-efficient way to guarantee the safety of subjects and the quality and integrity of clinical trial data.
Key Features
-
-
-
-
-
-
-
-
-
- Flexible definition of risk levels
- Straight-forward to implement and use
- Rules to define which parts of the CRF need to be verified for which patients
-
-
-
-
-
-
-
-
Double Data Entry (DDE)
Marvin supports double data entry both for paper and hybrid studies (combining paper and eCRFs). Marvin DDE consists of two independent Data Entry steps followed by automatic verification of correct data and discrepancies highlighted in a tabular overview. To ensure data entry quality control, a final review and entry approval.
Key Features
-
-
-
-
-
-
-
-
-
- Dedicated discrepancy reconciliation process
- Automated selection of matching forms, batch verification
- Attachable ‘memos’ for internal notes and workflow support
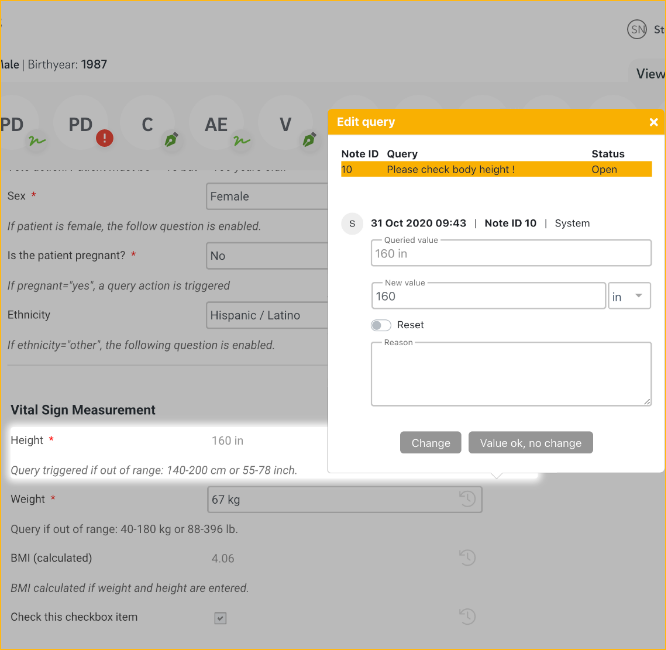
- Automated and manual query workflow including query printing
- Hybrid DDE/EDC mode by patient, by site or by page
-
-
-
-
-
-
-
-
Coder
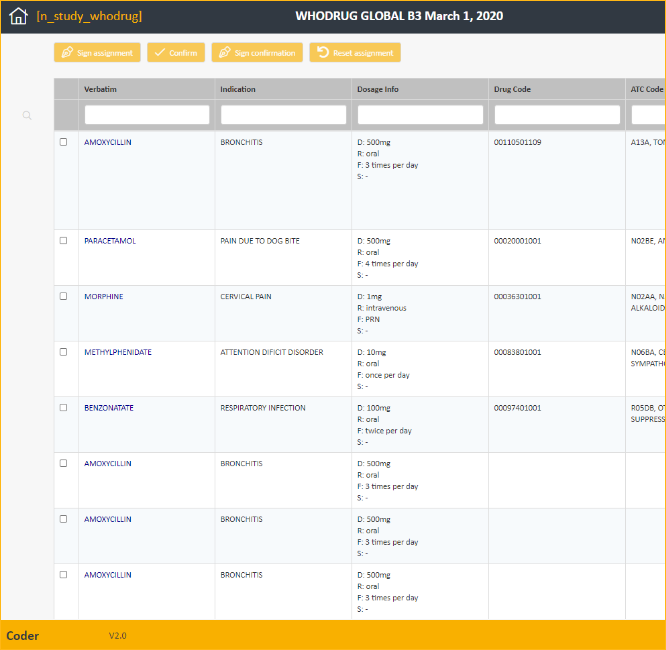
Classify safety-related data such as adverse events or concomitant medications. Coder supports standard MedDRA and WHODrug dictionaries to automatically and manually encode verbatim terms use of powerful search mechanisms. The coder module is fully integrated with Marvin via a Web Service API.
- Customizable standard user roles and workflows
- Intuitive user interface
- Upgrade dictionary during study conduct
Composer (eCRF Design)
With XClinical’s Composer, you can produce eCRF designs, document links, SDTM Tabulation and more to live in a single intuitive interface for your team. Never again struggle with any traditional office tool. The tool includes an SDTM reviewer as perfect add-on to visualize your SDTM data.
- Smart metadata library functionality
- Easy setup and management of amendments
- Automatic output of the annotated CRF, data validation plan and more
Reporter
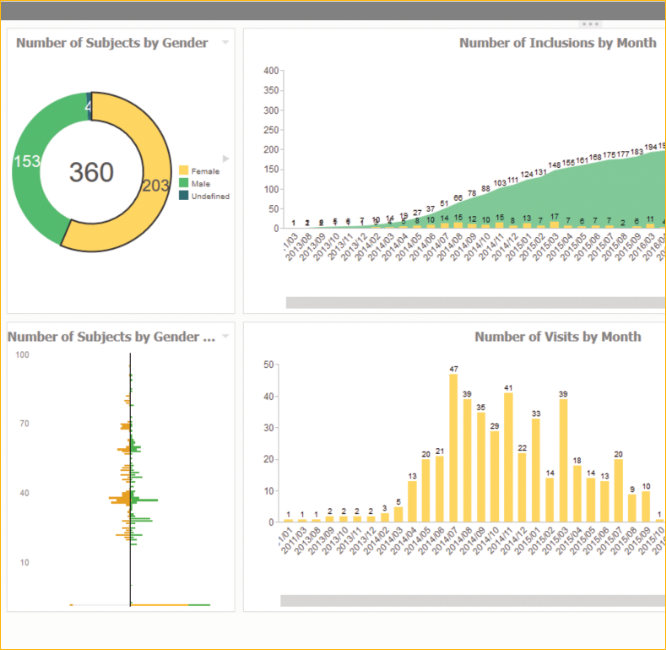
Marvin provides standard reports, custom reports, dashboards and interactive ad-hoc reports. The intuitive self-service reporting system enables users to create and publish their own reports without programming knowledge.
- Real-time data
- Linking of reports to the eCRF
- Pick any CRF data with a simple mouse click
- Report publishing in any format
Expert Ressources
Marvin is a universal software platform with multiple features to better manage the whole lifecycle of your clinical trial. Find out more.
 Features Overview
Features Overview
 Pricing & Licensing
Pricing & Licensing